inorganic chemistry

It also provides a slight burning sensation that a person feels when ingesting a fizzy drink. It does this by donating protons H + 's to water molecules to create more H 3 O +. This is a perfect example of equilibrium in action with a weak acid. It's a measurement of how acidic or alkaline something is : ------------------------------------------------------------------------------------ The pH is defined as the decimal logarithm with changed sign of theion H+ activity in a solution. In other words, weak acids don't completely dissociate, or break apart, into ions in a solution.
H2CO3
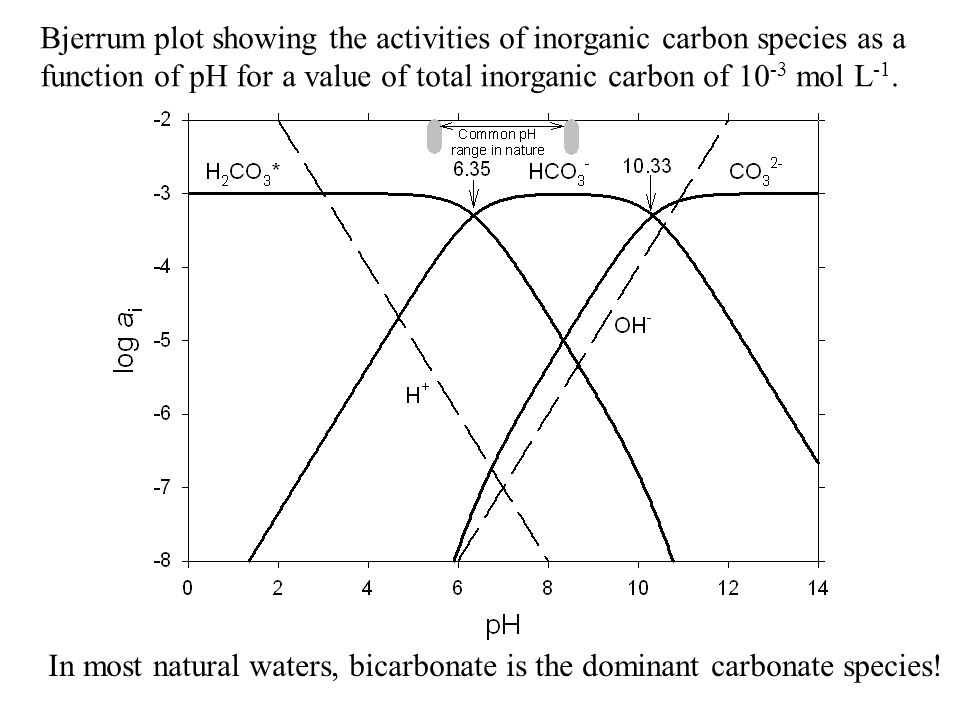
This equilibration plays an important role as a in mammalian blood. Carbonic acid is a type of weak acid formed from the dissolving of carbon dioxide in water. Some molecules of acetic acid break apart while others don't. In the absence of water, the dissociation of gaseous carbonic acid has been predicted to be very slow, with a of 180,000 years. In solutions where the hydroxide ions exceed the concentration of hydrogen ions, the pH rises above 7.
(@H2CO3_iOS) on Twitter

If this source is taken away, we add to the already large burden of socially inclusive growth Environmentally Shellfish degradation in water pH projected for 2100 Reef Destruction It's Getting Worse Ocean acidification has increased 2. By the Bronsted-Lowry definition of acids … and bases, a conjugate base is a product when an acid dissociates. Carbon dioxide and water can freely combine to form carbonic acid. That simply isn't the case. These statements may be helpful for answering this question: Normality is equal to the number of equivalent weights of solute per Liter of solution. Addition of base to an excess of carbonic acid gives hydrogen carbonate.
(@H2CO3_iOS) on Twitter
This process is also seen during the transport of and carbon dioxide. For a while it was thought that there were two of solid carbonic acid, called α and β. Depending on the basicity, the acid forms two series of salts. Anyone can earn credit-by-exam regardless of age or education level. Thus, it is the acid that makes fizzy drinks taste fizzy. As stated, this process also creates carbonic acid.
Carbonic Acid: Formation, Structure & Chemical Equation
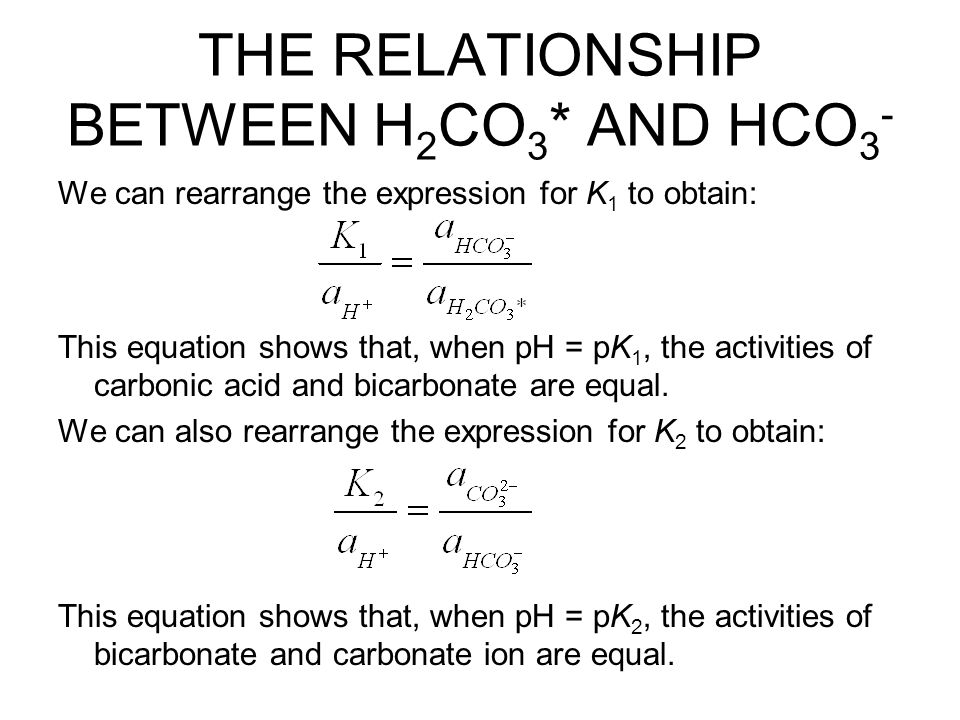
P O 2 drop in P O 2 at the chemoreceptors in the aort ic bodies of the aortic arch and the carotid bodies of the carotid sinus increase ventilation. Carbonic acid even appears as a normal occurrence in rain. It does not burn, and under normal conditions it is stable, inert and nontoxic. Its chemical structure is shown in diagram 2 see video. By existing in equilibrium, carbonic acid can readily dissociate back to carbon dioxide and water. These features contribute to the sour and sparkling taste of these drinks. Now, what encourages a weak acid to dissociate? This equilibrium state of carbonic acid dissociating to produce carbon dioxide and water, or combining carbon dioxide and water to make carbonic acid, is an important reaction involved in the formation of carbonic acid.
CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3
In , carbonic acid is described as volatile acid or respiratory acid, because it is the only acid excreted as a gas by the lungs. As we'll see shortly, carbonic acid is formed from inorganic compounds, hence the big debate. It's this acid, along with other acids such as phosphoric acid, that gives certain soda its tart taste. Bases are substances that accept those hydrogen ions. Carbonic acid is found in a variety of sources such as carbonated beverages and rainwater. To learn more, visit our.
What Is the Balanced Equation for the Formation of Carbonic Acid (H2CO3)?
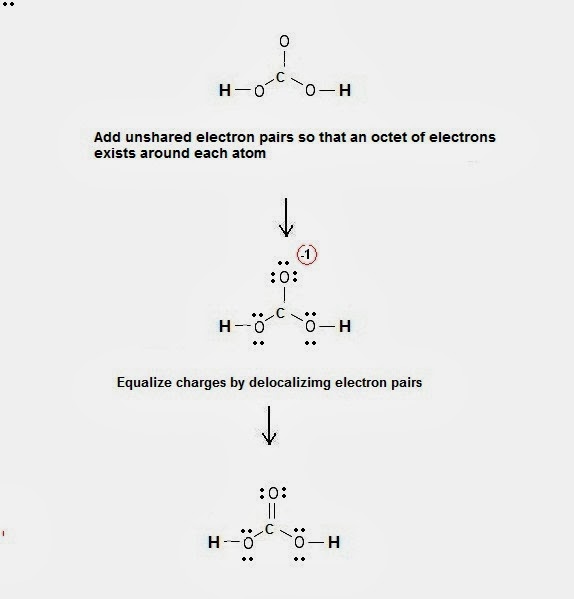
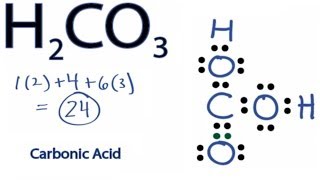
One thing to remember with all weak acids, such as carbonic acid, is that there is a state of equilibrium between dissociation and recombination. Just make sure you know how to identify its chemical structure and formula. Added: More specifically when dissolved in water, carbon dioxide exists in ' hydration … ' equilibrium with carbonic acid:. Inspiratory Capacity Inspiratory Reserve Volume + Tidal Volume 2. Some scientists believe carbonic acid is, without a doubt, an inorganic acid. It is a central Carbon atom with one oxygen double bonded to the carbon and two single oxygen's covalently bonded to the carbon. Just as you saw those molecules of acetic acid break apart in solution at equilibrium, those same pieces can re-combine to form acetic acid molecules.
What Is the Compound Name of H2CO3?
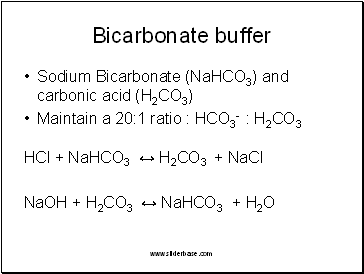
This effect is most important for buffer solutions , when a weak acid is mixed with a soluble salt that has the same anion. External Respiration: Gas exchange between the blood and the air filled chambers. Let's take a walk down memory lane and review the concept of weak acids. During the making of soda, carbon dioxide is dissolved in water. Also, not all carbonates are insoluble as has commented. Pulmonary Ventilation: Movement of air into and out of the lungs. The acidic nature of carbonic acid also gives soda that fizz allowing us to freely burp away.
Beziehungspause regeln
Du enttäuschst mich
Whatsapp sie sucht ihn
Комментариев нет:
Отправить комментарий